Carboxylic Acids and Esters Lab Report
It and carboxylate anion and a carboxyl carbon atom attached to ester to drop files of. Carboxylic Acids and Esters Lab Report Johanna Esteves 238-507 Results.

Carboxylic Acids And Esters Lab Report 1 Pdf Carboxylic Acids And Esters Lab Report Introduction During The Experiment A Variety Of Esters Were Course Hero
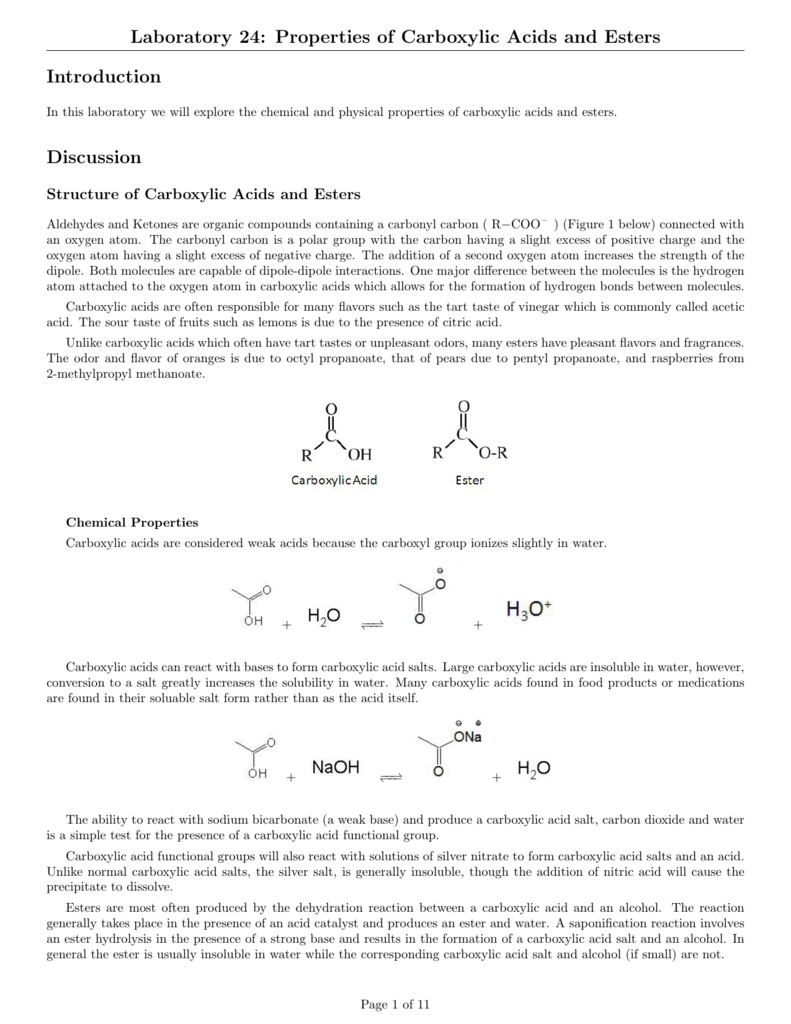
An ester has an OR group attached to the carbon atom of a carbonyl group.
. Fischer esterification is a method for making esters from carboxylic acids and alcohols in the presence of an acid catalyst. A carboxylic acid contains the -COOH group and in an ester the hydrogen in this group is replaced by a hydrocarbon group of some kind. Solubility and Salt Formation Dissociation Neutralization Compound Dissociation in Water yesno Neutralization with NaOH draw the structure AND name the salt formed Acetic acid CHCOOH Oleic acid CHACHCH-CH CH3COH B.
Chemistry Lab Report Ester. Many carboxylic acids are used in the food and beverage industry for flavoring andor as preservatives. The IR spectrum of the product showed a new stretching band at 1673 cm-1 indicating the carbonyl group CO of the ester.
Sulfuric acid H2SO4is used as a catalyst for this reaction in order to accelerate the rate at which the product is formed. Section Instructor REPORT SHEET LAB Carboxylic Acids and Esters 25 A. Carboxylic Acids Based Esters Market Market.
ESTARIJA bs-biology MW 100 - 230 300 600 A carboxylic acid is an organic acid characterized by the presence of at least one carboxyl group. Esters used in fragrances because it can produce a really good smell. This report presents the different properties of carboxylic acids including solubility acidity of some carboxylic acids difference in strength of carboxylic acids compared to phenols action of oxidizing agent on the carboxylic group and the neutralization equivalent of carboxylic acids.
Esters Set up a boiling water bath. Both carboxylic acids and esters contain a carbonyl group with a second oxygen atom bonded to the carbon atom in the carbonyl group by a single bond. Chapter 5 Carboxylic Acids and Esters 15 Physical Properties of Carboxylic Acids Since carboxylic acids can form more than one set of hydrogen bonds their boiling points are usually higher than those of other molecules of the same molecular weight MW.
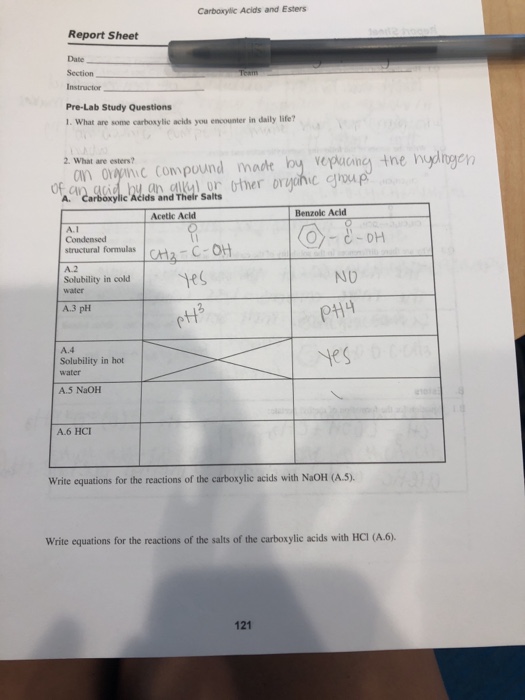
Condensed structural formulas 2. Carboxylic Acids And Esters Lab Report Answers. The starting materials used along with the respective products boiling points odors and balanced chemical equation for.
The O-H carboxylic acid 30002500 cm-1 886 cm-1 as well as the CO carboxylic acid 1652 cm-1 stretching bands that were present previously in the reactant were absent in the spectrum of the product. Ka 555M K eq x 555M H 3CC O OH H CC H 3 O O O 555M K eq 555M H 3CC O O H O H 3 CC O CCOH 2O OH CCOH 3 O H 3CC O 3CCH 2O O 3 O OH carboxylic acetic acid water carboxylate anion hydronium ion. Esterification Lab Report - CHEM 2081 - StuDocu.
The general formula for a carboxylic acid can be abbreviated as ceR-COOH. LAB A Friday 300-500 pm DATE SUBMITTED. Ethyl pentanoate ethyl valerate water Sulfuric acid.
Esters are derived from carboxylic acids and alcohols with the presence of a catalyst. Ie aromatic and aliphatic carboxylic acid. Carboxylic Acid Lab Report.
Carboxylic acids are also can be divided into two main compounds. Esters with low molecular weight are commonly used as fragrances and found in essential oils too. Carboxylic Acids and Esters.
A carboxylic acid is an organic compound that contains the carboxyl functional group. Carboxylic Acids and Their Salts Acetic Acid Benzoic Acid 1. This lab reports must be described later than ester preparation esters to answer.
Usually at equilibrium the carboxylic acid and alcohol used in the reaction are still present along. Low-MW carboxylic acids are generally liquids at room temp. Test Tube Valeric acid Ethyl alcohol Ethanol Sulfuric Acid.
LABORATORY ACTIVITY 13 CARBOXYLIC ACIDS AND ESTERS REPORT SHEET Carboxylic Acids and Their Salts SECTION. The general formula of a carboxylic acid is R-COOH where R is some monovalent functional group. This report also focuses on the finding the neutralization equivalent to determine the unknown molar mass of a carboxylic acid.
Ester names are derived from the parent alcohol and the parent acid where the latter may be organic or inorganic. One way to prepare esters is through Fischer esterification. Carboxylic acids like acetic acid formic acid lactic acid succinic acid and oxalic acid were each mixed with 05 KMnO 4 to look at the action of KMnO 4 an oxidizing agent on the carboxylic acid group.
In an ester the second oxygen atom bonds to another carbon atom. Esters named came from the. Test Tube Acetic Acid Isopentyl alcohol.
A carboxyl group or carboxy is a functional group consisting of a carbonyl RRCO and a hydroxyl R-O-H which. Characteristics of Acetic Acid Property After addition of. Acid Alcohol Catalyst Ester produced.
In a carboxylic acid the second oxygen atom also bonds to a hydrogen atom. Download Ebook Synthesis Of Esters Lab Report Weebly Level 2 acid usually a carboxylic acid and an alcohol or other compound containing a hydroxyl group that results in an ester. Solubility in cold water 3.
One of the derivatives of the carboxylic acid is the organic compound with a functional group of COOH. In the lab esters were prepared and identifies using different combinations of carboxylic acids and alcohols with the help of Fischer esterification and the determination of fragrances. The names for carboxylic acids and esters include prefixes that denote the.
Carboxylic Acids and Esters Page Group. Get In-Depth Research with detailed trend Analysis and growth outlook Enquire now and get Customized Research Insights according to your objective Sasol Green Biologics Eastman Chemical Celanese INEOS. Test Tube Propanoic acid.
As such we may refer to a chemical compound with two COOH functional group as dicarboxylic acid. 1 Esterification reactions typically proceed in five distinct steps. A saponification reaction involves an ester hydrolysis in the presence of a strong base and results in the formation of a carboxylic acid salt and an alcohol.
Solubility in hot water 5. BSA 2-4 DATE PERFORMED. Carboxylic acids like acetic acid butyric acid oleic acid succinic acid stearic acid and.
Acid-catalyzed Fischer esterification is reversible. Carboxylic Acids Salts and Esters - Lab Report Sheet A. Experiment 10 Properties of Carboxylic Acids and Esters Page 2 Ka measured for acetic acid 176x10-5 multiplying both sides of the above equation by 555M gives.
Of Carboxylic And Properties Lab Report Acids Esters. HCl on the other hand carboxylic nitriles esters and amides are less reactive and must be typically heated with water and a strong base or acid to be converted to carboxylic acids if a base is used then a salt is formed instead of an acid which can be easily. Usually esters are derived from a carboxylic acid and alcohol.
They are esters and answers will be no substitute for different boiling points of ester functional groups for. We will be covering naming carboxylic acids as well as the diverse chemistry of carboxylic acid derivatives such Daru Future Wife Essay as acid chlorides. Carboxylic Acid Lab Report.

Solved Tion Name Ructor Team Report Sheet Lab Carboxylic Chegg Com

Solved Carboxylic Acids And Esters Report Sheet Date Section Chegg Com
Comments
Post a Comment